Research
Medical images allow doctors to assess patient health by revealing anatomy without invasive procedures. For example, enlarged heart ventricles are signs of cardiac disease; the location of cerebral hemorrhages can indicate the location of arterial vessel wall rupture in the brain. Advanced, high-resolution medical images, when appropriately analyzed, even make it possible to extract functional information. For example, the pumping power produced by the heart can be measured by dynamic imaging; characteristics of blood flow in the brain can show the locations of weakened vessel walls before rupture occurs.
Our lab develops tools and algorithms for obtaining individualized diagnoses and information about organ function from patient medical images made during regular clinical evaluation. Therefore, a record of health changes can be compared on an individual basis without additional medical procedures. More importantly, it can help follow each person's response to treatments, including implantable devices and medications. We work closely with the clinical side of the Ronald Reagan UCLA Medical Center and assist the Division of Interventional Neuroradiology to evaluate the risk of brain aneurysm rupture. We also work with the Division of Interventional Radiology to evaluate the performance of inferior vena cava (IVC) filters and the flow effect of Transjugular Intrahepatic Portosystemic Shunt (TIPS) procedures. We also collaborate with industrial partners in innovative medical imaging and patient-specific implantable devices.

IS FlowMap and Multi-dimensional Medical Image Analyses
[2013-JNIS] [2015-JNIS] [2015-JVIR]
Blood flow information is critical to inform interventional procedures and treatment planning. We have developed new computer programs to help visualize flow changes based on standard angiograms (3 frames per second). Our pilot study showed that during the interventional procedure not every aneurysm case has the same blood flow improvement after stent implantation.

Patient-specific Hemodynamic Analysis to Predict Aneurysm Rupture
[2014-JNIS] [2013-Neuroradiology] [2009-Surgical Neurology]
Brain aneurysm growth and rupture has been repeatedly linked to hemodynamic properties. To understand the characteristics of high rupture risk blood flow, we have created hemodynamic models to calculate flow differences between ruptured and unruptured aneurysms. Analyzing 41 aneurysms from the same anatomical location, we found significant blood flow changes during the cardiac cycle in ruptured aneurysms.
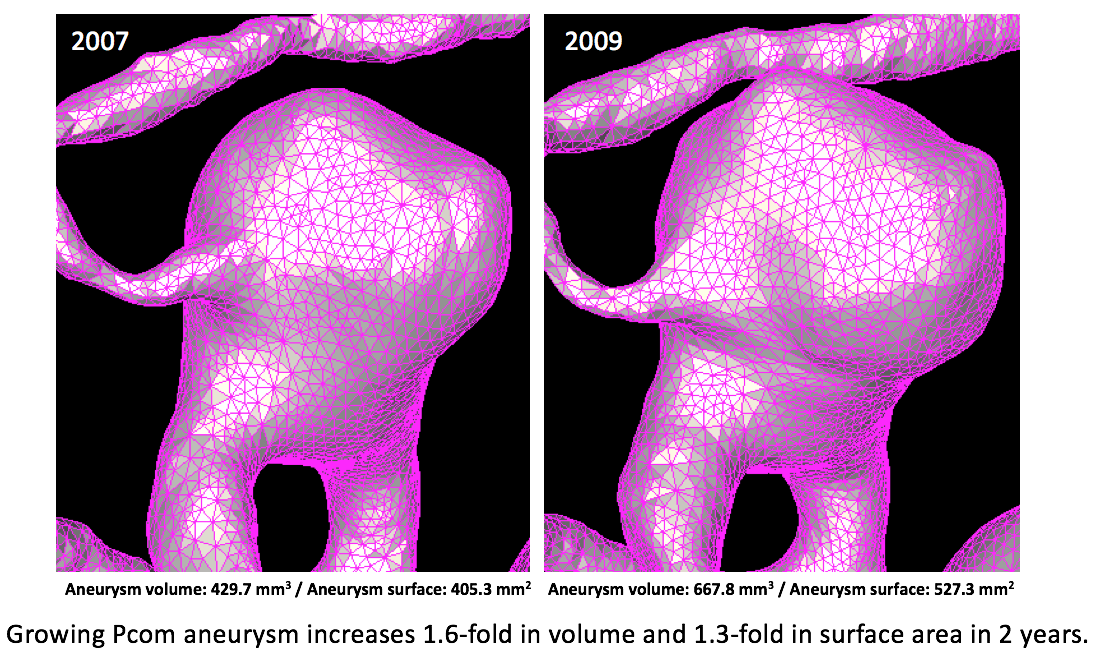
Disease growth analysis- 3D morphology analysis to quantify aneurysm growth
[2014-IEEE] [2013-JNS] [2013-Stroke] [2006-LSM]
Intracranial aneurysms affect 3-5% of the general population. Because aneurysm rupture can be very debilitating, it is important to understand the risks of rupture and see how unruptured aneurysms will behave over the long term. We studied the growth of more than 235 unruptured aneurysms and found that single and multiple aneurysms may have different growth risk factors. For those aneurysms which eventually ruptured, certain high risk morphology appeared (such as surface area change) while progressing toward rupture.
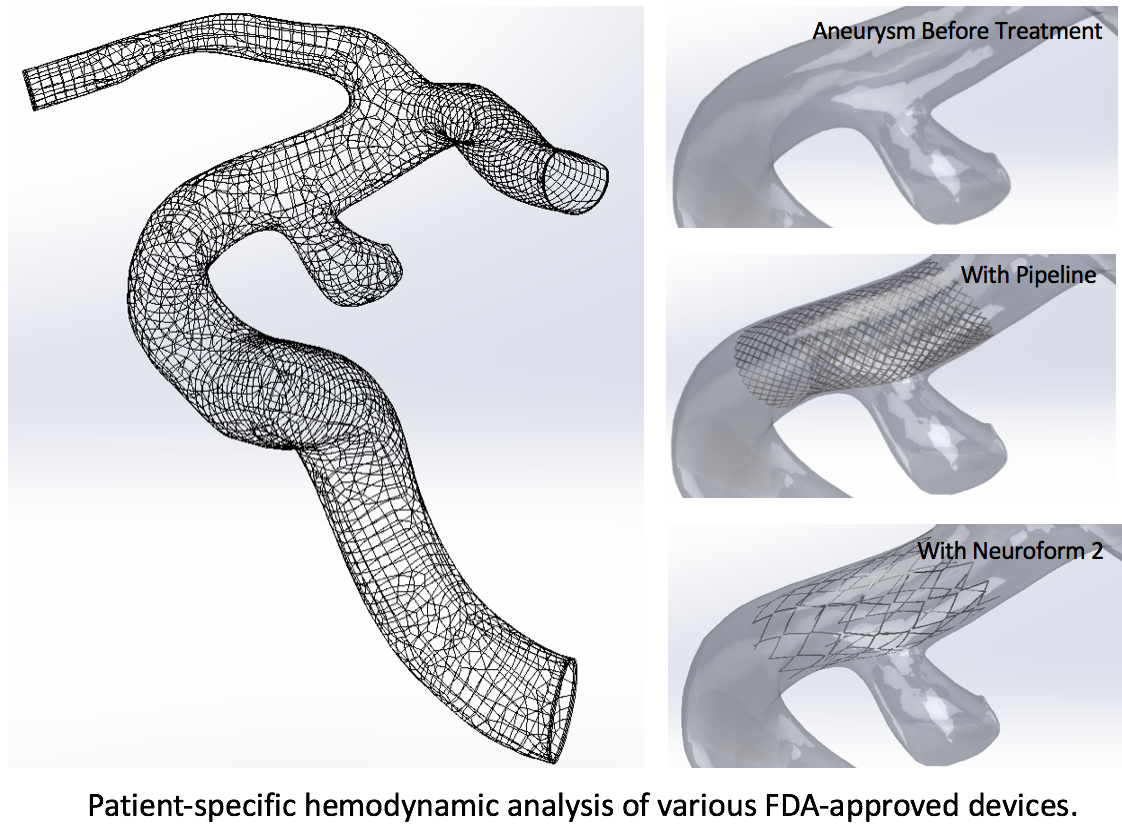
Medical devices simulation for personalized treatment planning
[2015-JVIR] [2015-NRJ] [2013-JNS] [2008-MICCAI]
Advanced medical devices have been used to help treat vascular disease. We used computer simulation combined with realistic patient image data to investigate the fundamental effectiveness of FDA approved devices, as well as create new designs to improve the biocompatibility of the devices. This technology also allows us to simulate the possible outcome of blood flow improvement with different devices for pretreatment planning for individual patients (such as intracranial stents or IVC filters).

Cardiovascular Simulation / Myocardium Soft Tissue Mechanics
[2007-WITTBH] [2004-JCF] [2004-MICCAI]
Our cardiac simulation provides information about myocardium function in addition to blood flow information. Using patient MRI images, we analyze the myocardium wall stress distribution during cardiac contraction. This virtual heart model not only helped understand the cardiac force distribution after myocardial infarction (MI), it also helped evaluate different pacemaker materials to find a mechanically optimal pacemaker lead which can reduce the impact on heart muscle for each patient.